What is a Nusantara Vaccine?
- 13, Sep 2021 14:59
- Heru
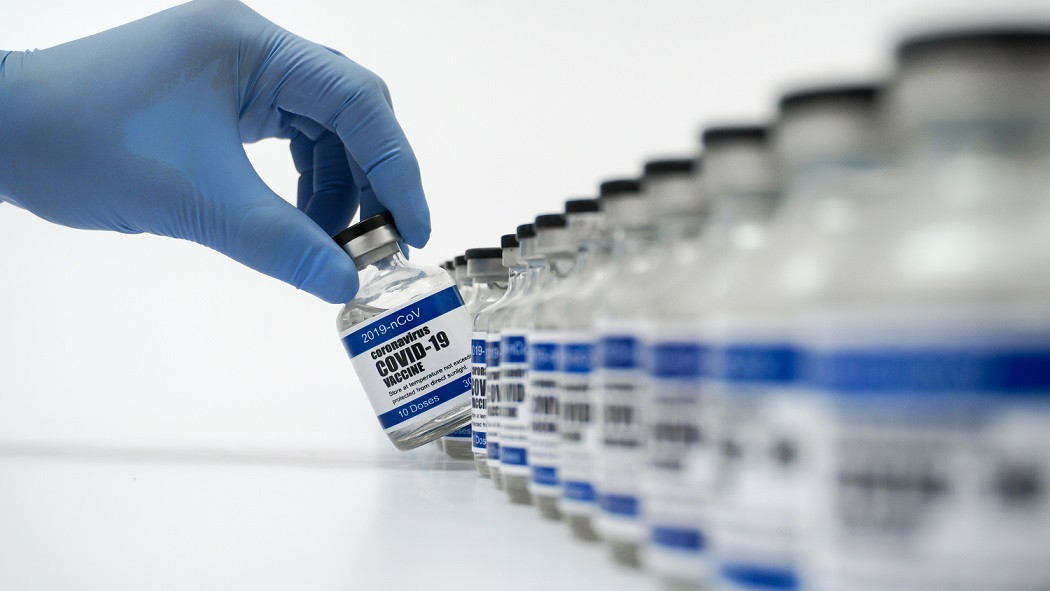
Recently, Nusantara Vaccines have often become a public concern. The Nusantara Vaccine is a vaccine that was initiated by the former Indonesian health minister, dr. Terawan Agus Putranto. The Nusantara Vaccine developed by the Indonesian Ministry of Health, Central General Hospital by Dr. Kariadi Semarang, and Diponegoro University for helping contribute to the overcoming COVID-19 pandemic. Currently, the Nusantara Vaccine has completed its phase 2 clinical trial.
Here are some facts about Nusantara Vaccines that you should know:
Manufacturing Process
The manufacturing process of the Nusantara Vaccine goes through a number of strict stages. The manufacturing process is done by taking a sample of the patient's blood, then the blood sample will be taken to the laboratory for the separation process between white blood cells and dendritic cells / defence cells (dendritic cells are part of white cells). This cell separation is done to make the dendritic cells able to recognize the covid-19 virus. After the cells have recognized the covid-19 virus, the dendritic cells will be taken back to be injected into the patient's body, which is already in the form of a vaccine. The manufacture of this vaccine is expected to be able to provide strong and good antibodies to prevent the viral infection into the human body.
Vaccine Based on Dendritic Cells
Quoted from kompas.com, the Nusantara Vaccine is claimed to be the first and only vaccine in the world that uses dendritic cells in the manufacturing process. The Nusantara Vaccine research team, Yetty Movieta Nency, said that the vaccine manufacturing process uses a dendritic cell approach in the aim to make the vaccine not contain coronavirus substances. The dendritic cells will contain a protein S antigen from the virus that causes COVID-19, i.e. SARS-CoV-2, so when it has been injected into the patient's body, the patient's antibody system will be stronger and immune from any viruses.
How it Works
The Nusantara Vaccine works is quite complicated because we have to remove and reinsert the dendritic cells from / to the human body. It works by taking a blood sample from the patient’s body, then separating the red blood cells from the white blood cells. This separation is done in order to grow dendritic precursor cells, these cells will be given a special compound to grow the dendritic cells. It takes an incubation period of 2-3 days before it is finally injected into the patient's body. During this incubation period, dendritic cells were added to contain the SARS-CoV-2 antigen.
Affordable Cost
The cost that we need to spend for this vaccine is only around Rp. 144,000 per patient. The shipping cost of this vaccine is also relatively low and affordable because it does not require special storage equipment, and the manufacture, processing raw materials is easy to send to many health facilities.
The Side Effects for Our Body
Quoted from kompas.com, the side effects of the Nusantara Vaccine are still relatively mild, such as soreness, bruising, redness, and itching. Most patients only complain about the soreness at the injection point and none of the patients need a special treatment due to the side effects.
Have Completed Phase 2 Clinical Trial
Recently, the Nusantara vaccine is claimed to have completed its Phase 2 clinical trial. Quoted by cnbcindonesia.com, in the phase 2, there are several requirements for patient subjects to be tested, such as a minimum age of 18 years, and understanding and agreeing to comply with the procedure. The vaccine research team also said that they will continue to evaluate the results of phase 1 and 2 clinical trials for about a year. This is to anticipate the side effects that have occurred in the patient's body.
Visit our website at hartechindonesia.com to complete your laboratory vaccination test equipment needs with our hi-tech covid-19 solutions products.
- 14, Sep 2022 13:43
- 29, Oct 2021 09:23
- 28, Oct 2021 09:53
- 27, Oct 2021 10:25
- 27, Oct 2021 10:20
- 26, Oct 2021 11:05
- 26, Oct 2021 11:00
- 25, Oct 2021 10:37
- 25, Oct 2021 10:32
- 22, Oct 2021 11:30
- 22, Oct 2021 11:27
- 22, Oct 2021 11:21
- 21, Oct 2021 11:58
- 21, Oct 2021 11:51
- 21, Oct 2021 11:44
- 21, Oct 2021 11:36
- 18, Oct 2021 13:02
- 15, Oct 2021 13:37
- 15, Oct 2021 13:33
- 15, Oct 2021 13:27
- 14, Oct 2021 10:58
- 14, Oct 2021 10:36
- 14, Oct 2021 10:31
- 14, Oct 2021 10:27
- 08, Oct 2021 12:11
- 08, Oct 2021 12:06
- 08, Oct 2021 12:01
- 07, Oct 2021 17:24
- 07, Oct 2021 09:57
- 06, Oct 2021 12:29
- 06, Oct 2021 12:22
- 05, Oct 2021 13:35
- 05, Oct 2021 13:30
- 01, Oct 2021 12:01
- 01, Oct 2021 11:44
- 30, Sep 2021 12:55
- 29, Sep 2021 13:48
- 28, Sep 2021 11:43
- 28, Sep 2021 11:38
- 28, Sep 2021 11:35
- 27, Sep 2021 12:21
- 24, Sep 2021 10:40
- 24, Sep 2021 10:37
- 24, Sep 2021 10:32
- 24, Sep 2021 10:27
- 24, Sep 2021 10:23
- 23, Sep 2021 21:42
- 23, Sep 2021 21:34
- 23, Sep 2021 21:29
- 23, Sep 2021 21:25
- 22, Sep 2021 11:10
- 22, Sep 2021 11:06
- 22, Sep 2021 10:44
- 22, Sep 2021 10:38
- 22, Sep 2021 10:24
- 20, Sep 2021 12:09
- 20, Sep 2021 11:53
- 20, Sep 2021 11:45
- 17, Sep 2021 10:16
- 17, Sep 2021 10:10
- 17, Sep 2021 10:02
- 17, Sep 2021 09:51
- 17, Sep 2021 09:46
- 14, Sep 2021 12:45
- 14, Sep 2021 12:35
- 14, Sep 2021 12:29
- 14, Sep 2021 12:25
- 14, Sep 2021 12:09
- 13, Sep 2021 14:54
- 13, Sep 2021 14:07
- 13, Sep 2021 14:01
- 09, Sep 2021 16:16
- 09, Sep 2021 16:07
- 09, Sep 2021 15:57
- 09, Sep 2021 15:52
- 02, Sep 2021 09:24
- 02, Sep 2021 08:29
- 01, Sep 2021 08:49
- 01, Sep 2021 08:33
- 31, Aug 2021 08:46
- 31, Aug 2021 08:25
- 30, Aug 2021 09:40
- 30, Aug 2021 09:20
- 27, Aug 2021 08:52
- 27, Aug 2021 08:14
- 26, Aug 2021 12:08
- 26, Aug 2021 12:00
- 25, Aug 2021 08:32
- 25, Aug 2021 08:17
- 19, Aug 2021 08:37
- 19, Aug 2021 08:19
- 17, Aug 2021 08:40
- 17, Aug 2021 08:27
- 16, Aug 2021 08:31
- 12, Aug 2021 08:57
- 12, Aug 2021 08:41
- 11, Aug 2021 09:40
- 11, Aug 2021 08:50
- 10, Aug 2021 09:05
- 10, Aug 2021 08:47
- 09, Aug 2021 09:46
- 09, Aug 2021 09:08
- 05, Aug 2021 17:19
- 05, Aug 2021 16:08
- 18, Sep 2020 01:54
- 18, Sep 2020 01:50
- 18, Sep 2020 01:48


















































































.jpg)




























.jpg)







.jpg)





